Newly designed primers for FeFehydrogenases indicated that (i) fermenters, acetogens, and undefined species in a fen harbor hitherto unknown hydrogenases and (ii) Clostridium and Thermosinusrelated primary fermenters, as well as secondary fermenters related to sulfate or iron reducers might be responsible for hydrogen production in the fen Comparative analysis of FeFehydrogenase In FeFehydrogenases, catalysis takes place at a unique diiron centre (the 2Fe subsite), which contains a bridging dithiolate ligand, three CO ligands and two CN – ligands 6, 7 As a member of the wwPDB, the RCSB PDB curates and annotates PDB data according to agreed upon standards The RCSB PDB also provides a variety of tools and resources Users can perform simple and advanced searches based on annotations relating to sequence, structure and function These molecules are visualized, downloaded, and analyzed by users who

O2 Reactions At The Six Iron Active Site H Cluster In Fefe Hydrogenase Journal Of Biological Chemistry
Is layolis fe the same as kaitlib fe
Is layolis fe the same as kaitlib fe-The design of these catalysts takes much inspiration from hydrogenase enzymes (H 2ases) because they perform just such chemistry in nature (1–4) These enzymes come in three distinct varieties, as determined by the metals at their active sites NiFe H 2ases, FeFe H 2ases, and Fe H 2ases (Fig 1) (5–7) The H 2ases can be classifiedAnd Fe H2ases, which catalyze H− transfer Modeling these




The Crystal Structure Of Fe Hydrogenase Reveals The Geometry Of The Active Site Science
Fe−Fehydrogenases are a class of metalloenzymes that catalyze the production of H2 from two protons and two electrons In this work, we used density functional theory (DFT) calculations to analyze the mechanism of hydrogen production, providing insight into the role of the intermediates in the catalysis We also validated the exchangecorrelation functional applied within DFT forFeFehydrogenase Hcluster (the active group of DdH) reactivation Low spin states (singlet and doublet) and low oxidation states (I and II) have been used for the diirons 10, 19 in agreement with the experimental and computational data The electronic structure of the hydrogenase Hcluster (without proximal cubane) has been deterHydrogenase3 HydG has been shown to catalyze the cleavage of tyrosine to produce pcresol,4 and we have recently demonstrated that this tyrosine cleavage leads to formation of CN5 We report here that HydG also catalyzes the formation of CO, monitored via binding of CO to deoxyhemoglobin These results provide the first
For FeFehydrogenase Hcluster 1, (Scheme II, 1 → 2'), the HOMO is more diffused over Fe d, DTMA bidentate ligand, and over the exogenous ligand, ie, OH In spite of greater e orbital diffusion over the DTMA ligand, the H becomes captured by OH , for it is a stronger base than the N of the DTMA bridge, as the NBO charges The active site (Hcluster) of FeFehydrogenases is a blueprint for the design of a biologically inspired H 2 producing catalyst The maturation process describes the preassembly and uptake of the unique 2Fe H cluster into apohydrogenase, which is"The FeFehydrogenases are highly evolved catalysts Under optimum conditions, each molecule of the D desulfuricans enzymes can produce 9,000 molecules of hydrogen per second at 30ºC;
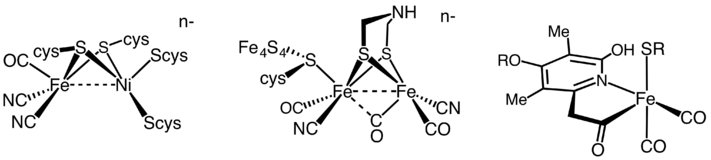
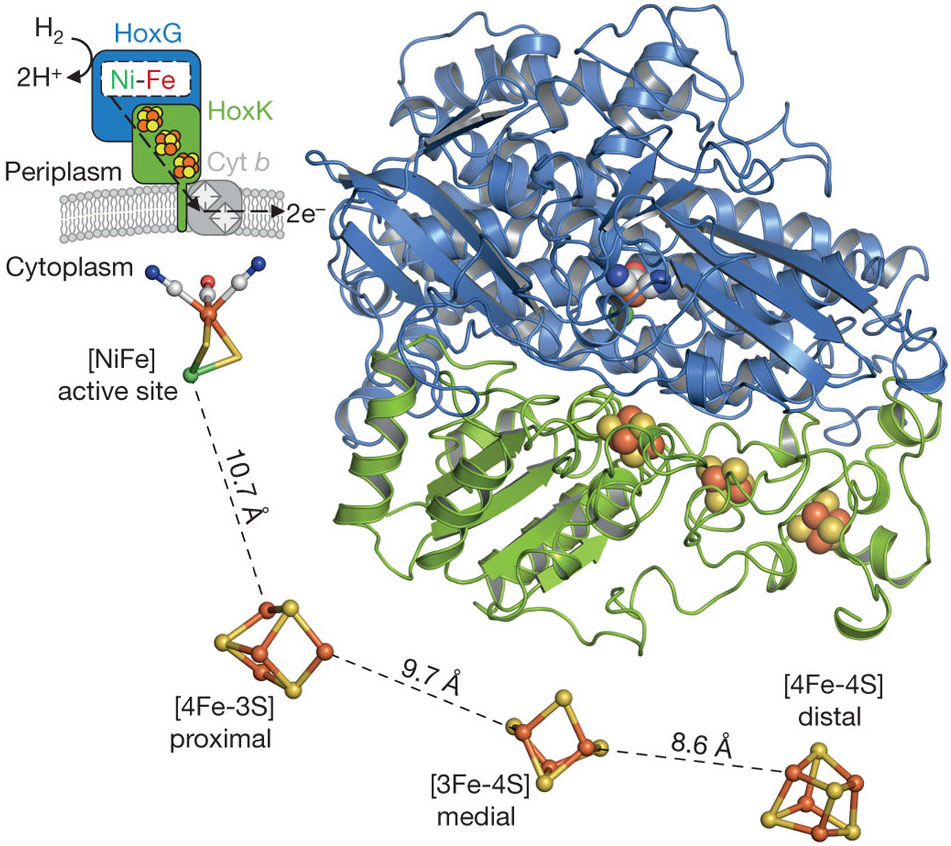
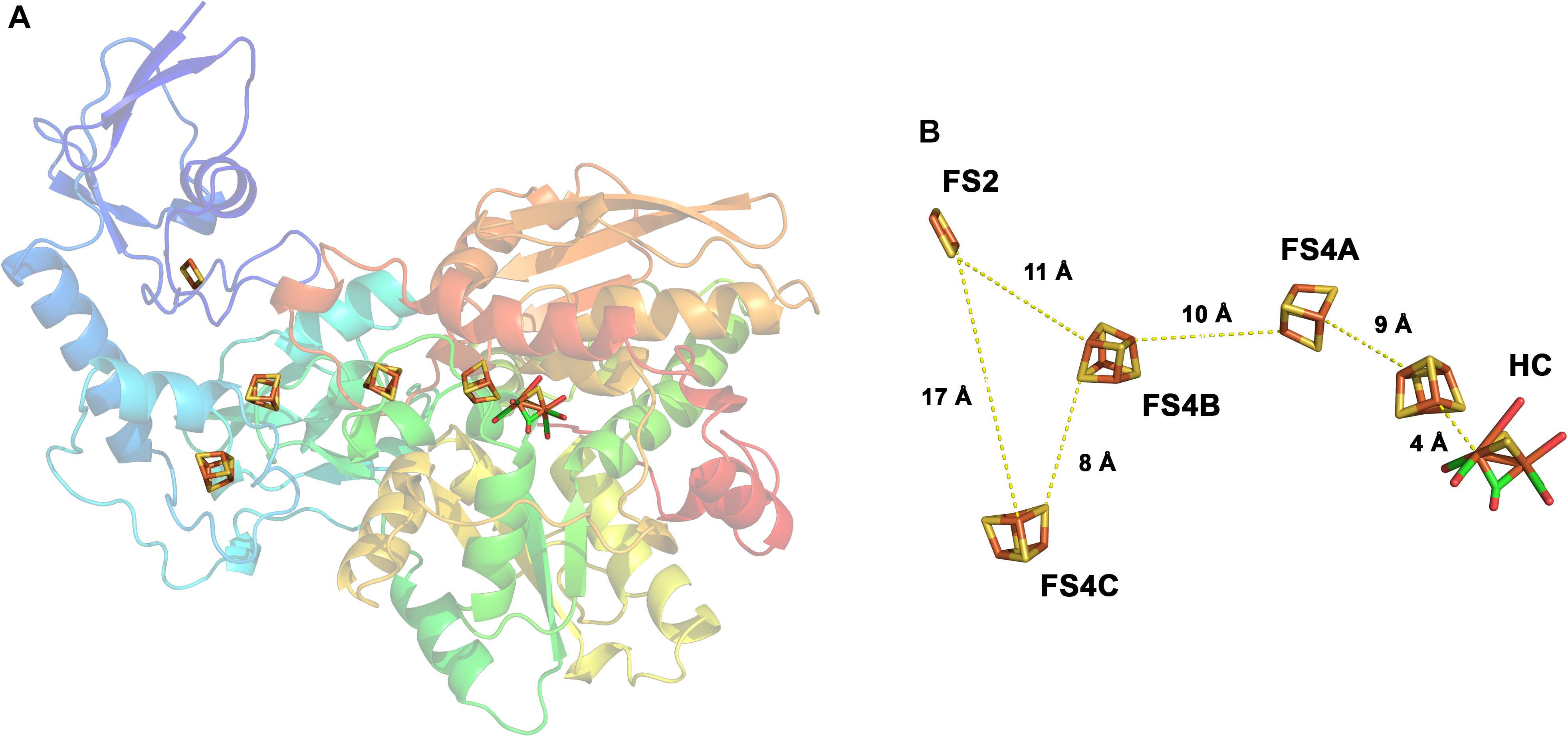
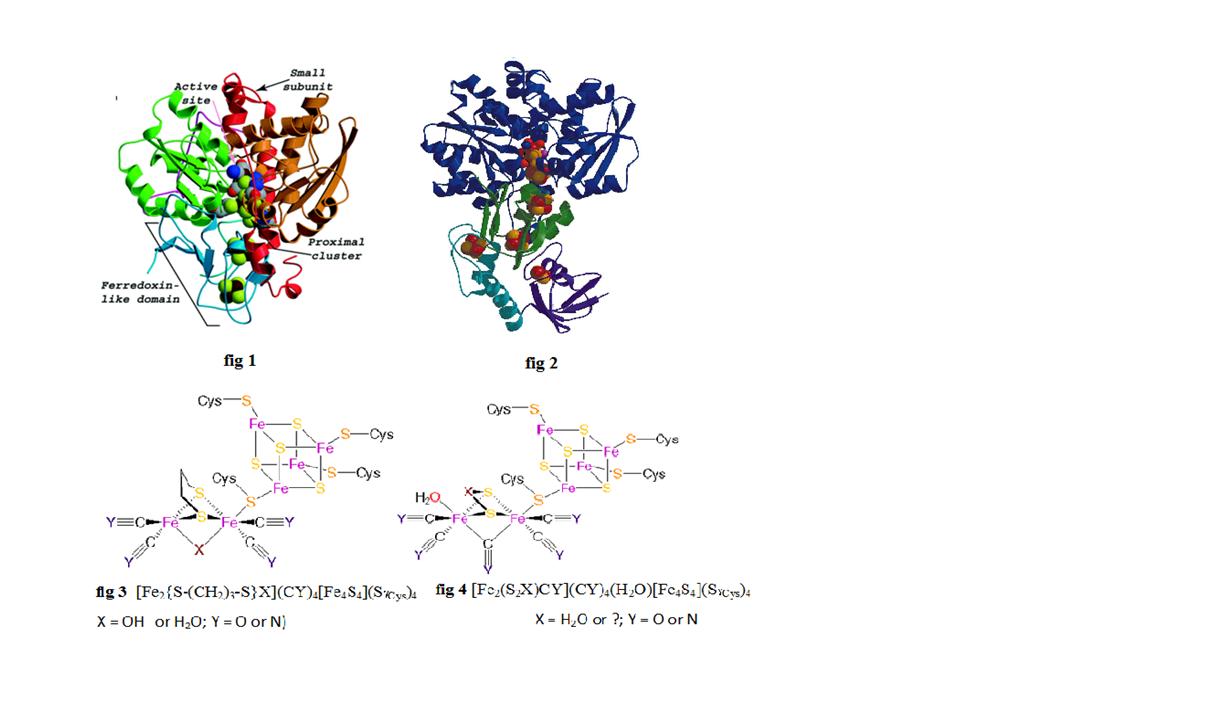
The threedimensional structures of FeFehydrogenase and NiFehydrogenase reveal the presence of a 4Fe4S cluster coordinated by a histidine residue, through the Nϵ atom in the first case and the Nδ atom in the second case (2, 1) Hydrogenases are difficult to investigate spectroscopically due to the large number of Fe atoms andTRIR spectra for the propylbridged FeFe(CO) 6Hydrogenase model compound in nhexane at 293 K using a) 532 nm pump, b) 355 nm pump, IR probe spectra, and c) steadystate FTIR spectrum of the compound for referenceFeFehydrogenase (from Chlamydomonas reinhardtii) is a metalloenzyme of interest due to its capability to reversibly catalyze the reduction of protons to molecular hydrogen with high efficiency (hydrogen metabolism) (10) Hydrogen metabolism produces ATP, which is an energy source for carrying out other metabolic processes



Www Jstor Org Stable



New Insights Into Fefe Hydrogenase Activation And Maturase Function
Hydrogenase Hydrogenases and Alternative Energies In order to decrease our dependency on fossil fuels, it is necessary to move to an energy economy that is based on alternatives to petroleum In this respect, hydrogen is the ultimate clean fuel and its use as a primary energy source desirable 1 It is well known that hydrogen could beAnd Fe H 2 ases, which catalyze Htransfer Modeling these enzymes has so far The study of hydrogenase enzymes (H2ases) is necessary because of their importance to a future hydrogen energy economy These enzymes come in three distinct classes NiFe H2ases, which have a propensity toward H2 oxidation;



Www Jstor Org Stable




Fefe And Nife Hydrogenase Diversity Mechanism And Maturation Sciencedirect
Be the first to rate this page As you found this page useful Follow us on social media!Electrocatalytic FeFehydrogenase mimics for the hydrogen evolution reaction (HER) generally suffer from low activity, high overpotential, aggregation, oxygen, of FeFehydrogenase Hcluster) was screened In the screening process, polar residues were removed, one at a time, and frequency calculations provided the change in Gibbs' energy of water dissociation (due to their



Core Ac Uk Download Pdf Pdf



Hydrogenase Wikipedia
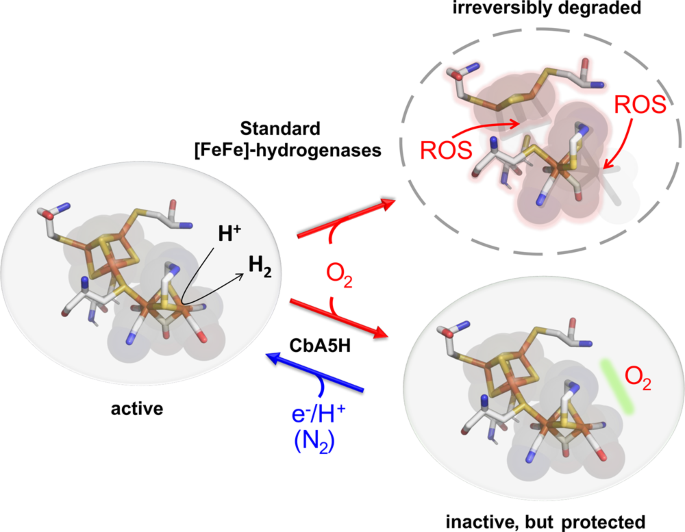
The oxidation of Hcluster in gas phase, and in aqueous enzyme phase, has been investigated by means of quantum mechanics (QM) and combined quantum mechanicsmolecular mechanics (QM/MM) Several potential reaction pathways (in the abovementioned chemical environments) have been studied, wherein only the aqueous enzyme phase has been found to lead to an We demonstrate that the insertion of the dinuclear active site of FeFe hydrogenase into the apoenzyme can occur when the enzyme is embedded in a film of redox polymer, under conditions of mediated electron transfer The maturation can be monitored by electrochemistry, and is as fast as under conditions of direct electron transfer The team used the FeFe hydrogenase from Desulfovibrio desulfuricans, a type of sulfatereducing bacteria The researchers found a sulfur ligand in the H inact state at the site where oxygen would attack (pictured) This prevents



Fe Only Hydrogenase Chemistry Libretexts




Frequency And Potential Dependence Of Reversible Electrocatalytic Hydrogen Interconversion By Fefe Hydrogenases Pnas
The NiFe and FeFehydrogenases catalyze the activation of H2 through the reversible reaction, H 2 ⇆ 2H 2e −, and function to either couple H 2 oxidation to energyyielding processes or reduce protons as a mechanism to recycle reduced electron carriers that accumulate during fermentation (Vignais and Billoud, 07) The simple organometallic, (μS2)Fe2(CO)6, serves as a precursor to synthetic analogues of the chemically rudimentary irononly hydrogenase enzyme active site The fundamental properties of the (μSCH2CH2CH2S)Fe(CO)32 compound, including structural mobility and regioselectivity in cyanide/carbon monoxide substitution reactions, relate to the Feonly Hydrogenase is a very important area of research Understanding the mechanism of Feonly Hydrogenase could open the doors for energy sciences development New developments in the research of Feonly hydrogenase has peaked interest for use of enzymes in the production of hydrogen




The Hydg Enzyme Generates An Fe Co 2 Cn Synthon In Assembly Of The Fefe Hydrogenase H Cluster Science




Activated Fe Hydrogenase Structure Nature Portfolio Chemistry Community
Appended FeFe hydrogenase mimic complex 111 The cage serves adual role, as it siteisolates the catalyst in addition to acting as the photosensitizerSelfassembled tetrahedral cage Fe4(ZnL)6 composed of six zinc porphyrinligands connected by four iron(II) corners is soluble in organic solvents and hasaGolbeck, John H In Photosynthesis research, Vol 143, No 2, , p Research output Contribution to journal › Article › peerreviewView protein in InterPro IPR, 2Fe2S_ferredoxinlike_sf IPR, 2Fe2S_ferredoxintype IPR0176, 4Fe4S_FeSbd IPR, 4Fe4S_Fe_S_CS IPR, Fe_hydrogenase IPR, Fe_hydrogenase_lsu_C IPR, Fe_hydrogenase_ssu IPR, Fe_hydrogenase_ssu_sf IPR, NADH_UbQ_OxRdtase_Gsu_4Fe4Sbd Pfam i



Hydrogenases




Ferredoxin Hydrogenase Wikipedia
The study of hydrogenase enzymes (H 2 ases) is necessary because of their importance to a future hydrogen energy economy These enzymes come in three distinct classes NiFe H 2 ases, which have a propensity toward H 2 oxidation;Fermentative H2production is catalyzed by FeFehydrogenases and group 4 NiFehydrogenases in obligate anaerobes (eg, Clostridiales) and facultative aerobes (eg, Enterobacteriaceae), respectively, functional groups that might respond differently to contrasting redox conditions FeFehydrogenase (FeFeH 2 ase) mimics, inspired by hydrogenases in nature, have emerged as an important class of molecular catalysts toward hydrogen (H 2) evolutionHerein, a per6thiolcyclodextrin (CDSH) engineered FeFeH 2 ase mimic/CdSe quantum dot (QD) assembly is established for effective photocatalytic H 2 evolution In the assembly, CDSH is first



Hydrogenase Inspired Catalysts Lichtenberger Research Group



Frontiers Complex Multimeric Fefe Hydrogenases Biochemistry Physiology And New Opportunities For The Hydrogen Economy Microbiology
FeFeHydrogenases are the most efficient enzymes for catalytic hydrogen turnover Their H 2 production efficiency is hitherto unrivalled However, functional details of the catalytic machinery and possible modes of application are discussed controversiallyThe enzyme CaHydA, a homologue (70% identity) of the FeFehydrogenase from Clostridium pasteurianum, CpI, was adsorbed to a negatively charged, selfassembled monolayer (SAM) for investigation by electrochemical scanning tunneling microscopy (ECSTM) techniques and macroscopic electrochemical measurements After confirming hydrogenase activity in the lysate (see Supplementary Table S4), the sandwich immunoassay configurations recognizing the C acetobutylicum FeFe hydrogenase were investigated



2




Pdf The Birth Of The Hydrogenase Studying The Mechanism Of Fefe Hydrogenase Maturation Semantic Scholar
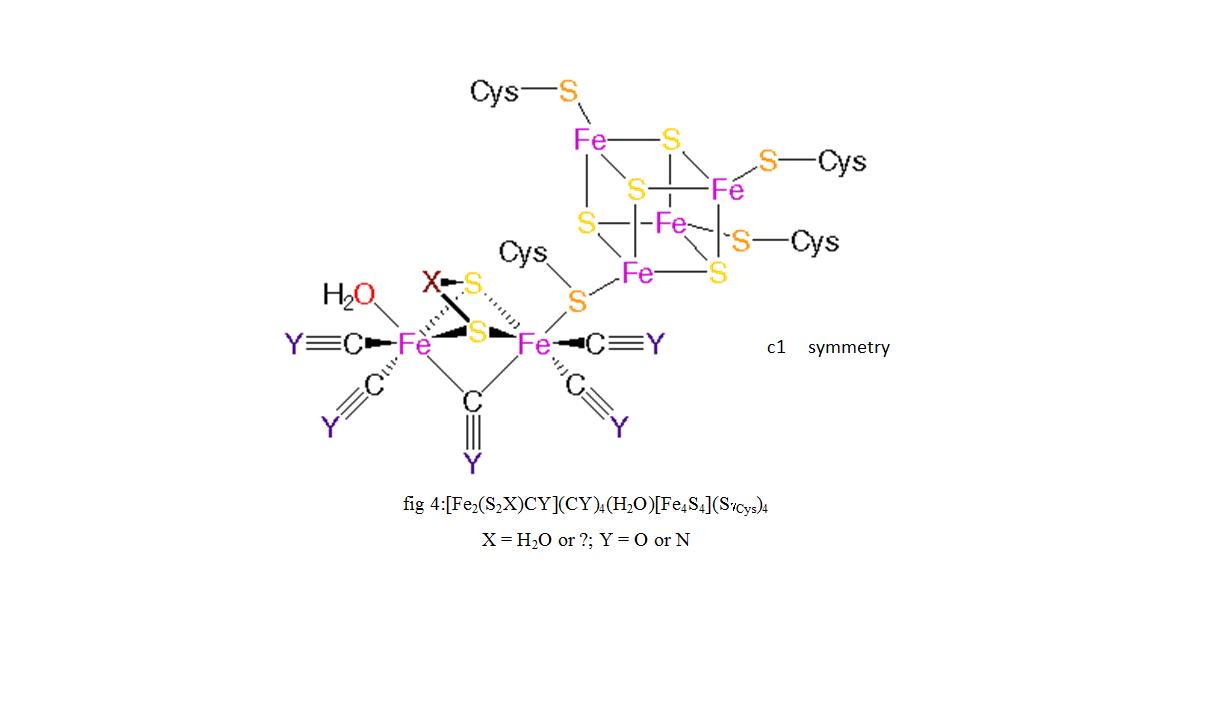
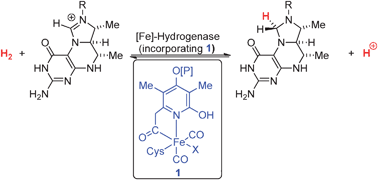
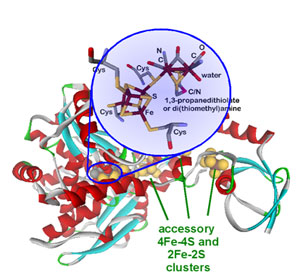
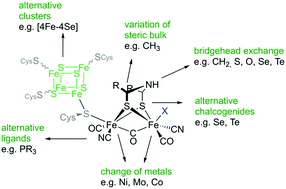
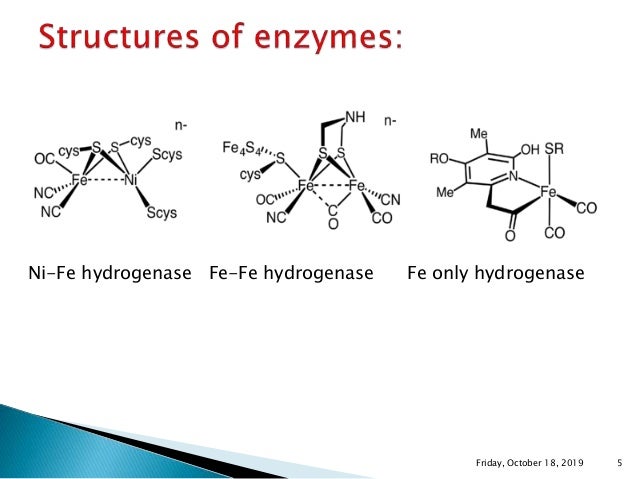
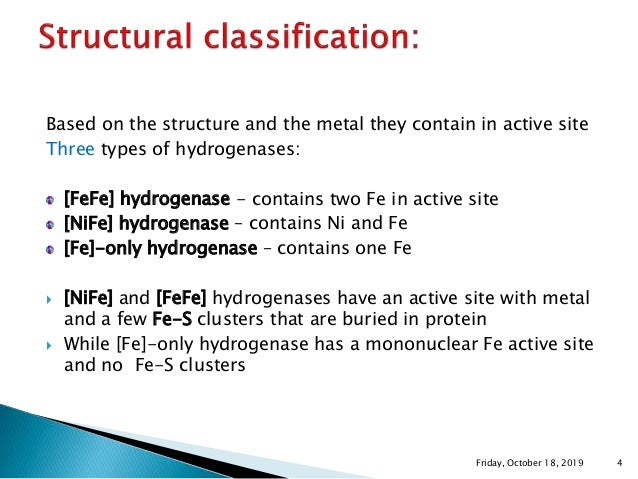
An unresolved structural issue for FeFehydrogenases is the nature of the dithiolbridging ligand in the diiron subcluster of the active site The two most probable candidates are 1,3dithiopropane (propane dithiol, PDT) and di(thiomethyl)amine (DTN) In the latter case, the dithiolbridging ligand is assumed to play a major role in the reaction cycle We report densityfunctional theoryClick on a star to rate it!Whereas most NiFe hydrogenases are of the hydrogen uptake type, FeFe hydrogenases mainly produce molecular hydrogen, and Fe hydrogenases catalyze a specific reaction utilizing H2 All hydrogenases contain at least one iron atom in the active site, which carries nonprotein ligands, ie, carbon monoxide and cyanide



1



Scholarworks Montana Edu Xmlui Bitstream Handle 1 28 Boyd a Mcr 14postprint A1b Pdf Sequence 1
Vignais and Colbeau, 04) NiFe hydrogenases have been found in bacteria and archaea, The eukaryotic green alga, Chlamydomonas reinhardtii, produces H2 under anaerobic conditions, in a reaction catalysed by a FeFe hydrogenase HydA1 For further biochemical and biophysical studies a suitable expression system of this enzyme should be found to overcome its weak expression in the host organism Two heterologous expression systems usedHydrogenases are widespread in nature and can be found in all domains of life Based on their phylogeny, they can be classified into three distinct classes that are named by the metal ions contained in their active sites as NiFe, FeFe, and Fe hydrogenases (Vignais et al, 01;



How An Oxygen Tolerant Hydrogenase Protects Itself From Oxygen



Frontiers Complex Multimeric Fefe Hydrogenases Biochemistry Physiology And New Opportunities For The Hydrogen Economy Microbiology
FeFe hydrogenase FeFe hydrogenase contains FeS clusters in their active site It is the fastest known biological catalysts for hydrogen oxidation and reduction but highly sensitive to oxygen and get inactivated in aerobic environment FeFe hydrogenase is also reported in few eukaryotes (Vignais and Billoud, 07)Submit Rating Average rating 1 / 5 Vote count 1 No votes so far!The promise of efficient, economic and renewable H 2 photoproduction from water can potentially be met by green algae These organisms are able to functionally link photosynthetic water oxidation to the catalytic recombination of protons and electrons to generate H 2 gas through the activity of the hydrogenase enzyme Green algal hydrogenases contain a unique metallocatalytic H




The Organometallic Active Site Of Fe Hydrogenase Models And Entatic States Pnas




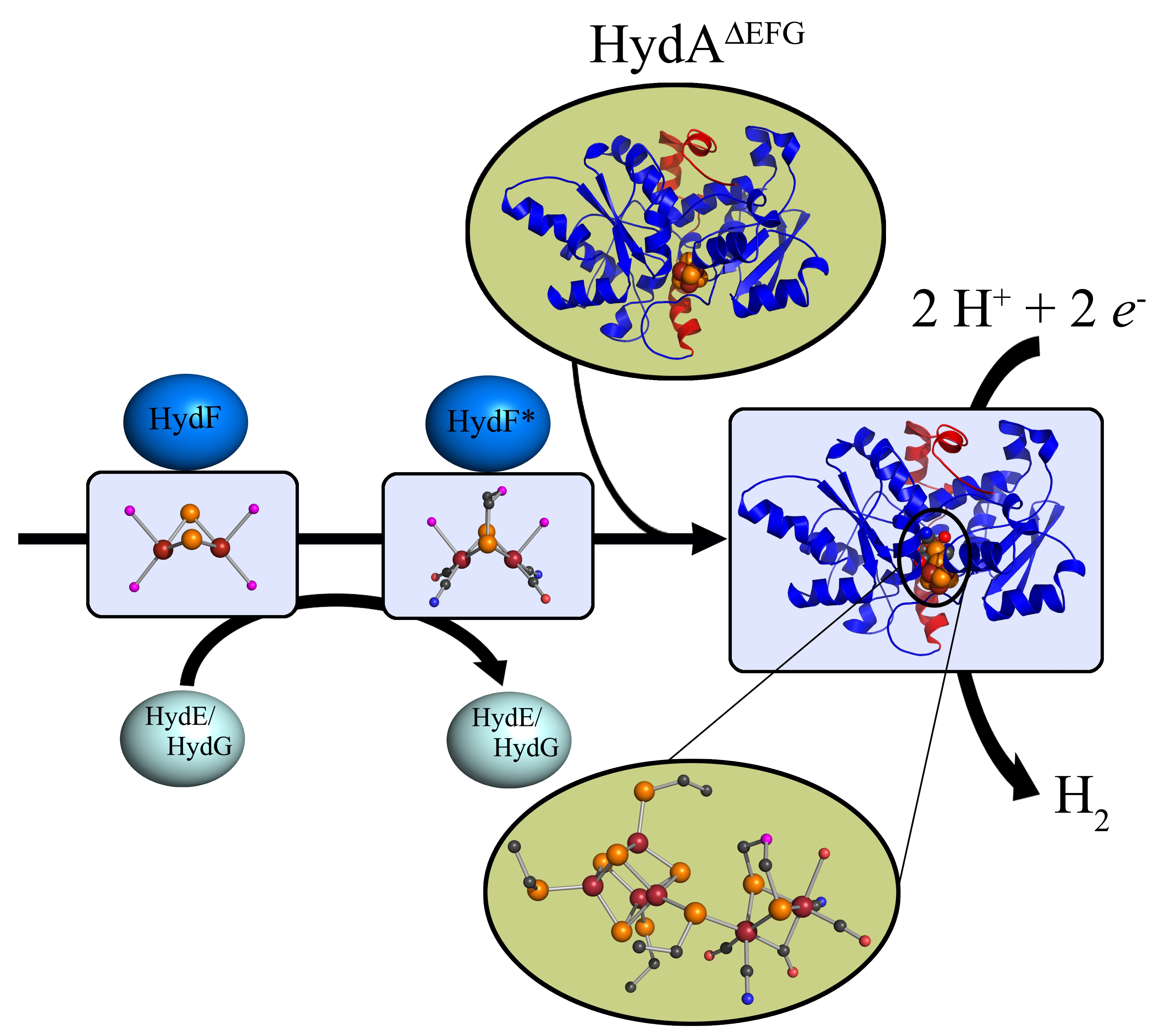
The Maturase Hydf Enables Fefe Hydrogenase Assembly Via Transient Cofactor Dependent Interactions Journal Of Biological Chemistry
Interestingly, and perhaps fortuitously, the timedependent DFToptimized exitedstate geometry of Fe 2(μS 2C 3H 6)(CO) 6 with a semibridging CO is reminiscent of the geometry of the Fe 2S 2 subcluster of the active site observed in FeFe hydrogenase Xray crystal structuresFeFe H 2 ases, which have a propensity toward H 2 evolution;For C pasteurianum hydrogenase the figure is 6,000 s 1



Hydrogenase Inspired Catalysts Lichtenberger Research Group




O2 Reactions At The Six Iron Active Site H Cluster In Fefe Hydrogenase Journal Of Biological Chemistry
Generating dihydrogen by tethering an FeFehydrogenase via a molecular wire to the A 1A /A 1B sites of photosystem I / Gorka, Michael;FeFe H2ases, which have a propensity toward H2 evolution;View protein in InterPro IPR, 2Fe2S_ferredoxinlike_sf IPR, 2Fe2S_ferredoxintype IPR0176, 4Fe4S_FeSbd IPR, 4Fe4S_Fe_S_CS IPR, Fe_hydrogenase IPR, Fe_hydrogenase_lsu_C IPR, Fe_hydrogenase_ssu IPR, Fe_hydrogenase_ssu_sf IPR, Fe_hydrogenase_subset IPR, NADH




Synthesis Of The 2fe Subcluster Of The Fefe Hydrogenase H Cluster On The Hydf Scaffold Pnas



Core Ac Uk Download Pdf Pdf
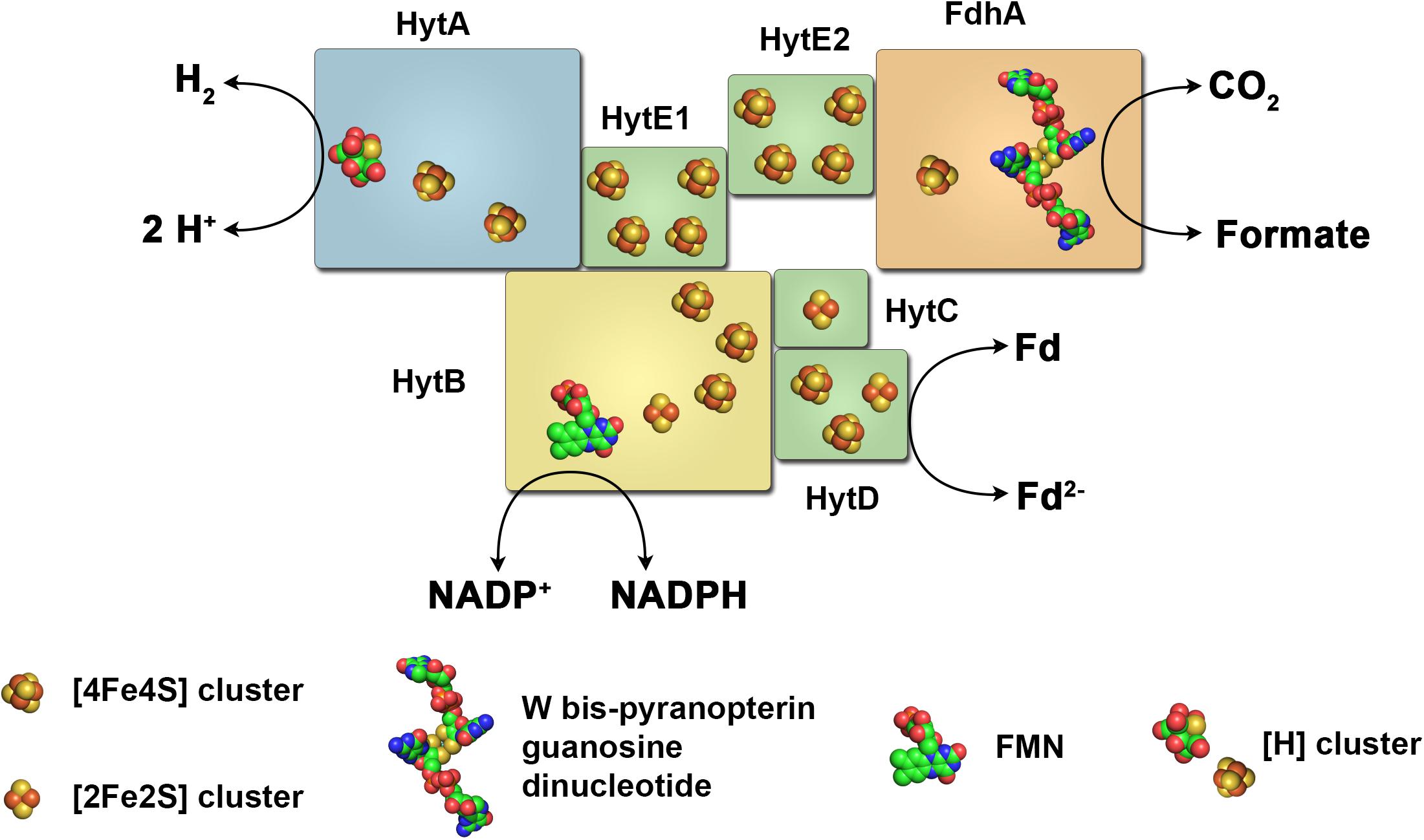
Hydrogenase from T maritima was shown to require both reduced Fd and NADH for H2 production 23 Conversely, the FeFehydrogenase from Acetobacterium woodii simultaneously reduces NAD and Fd during H2 oxidation The key to the reversibleHome / Gallery / Fe Fehydrogenase Fe Fehydrogenase CONTROLS 1 (1) How useful was this page?Hydrogenase Ca1and CdSe nanocrystals were used to study the mechanism of electron injection into FeFehydrogenase and the activation thermodynamics of the initial step of proton reduction (Figure 2) Compared to the algal CrHydA1 enzyme, Ca1 contains additional FeS clusters (Fclusters) that function in electrontransfer to the Hcluster




Molecular Basis Of Fefe Hydrogenase Function An Insight Into The Complex Interplay Between Protein And Catalytic Cofactor Sciencedirect




Learning From Nature Understanding Hydrogenase Enzyme Using Computational Approach Qiu Wires Computational Molecular Science Wiley Online Library
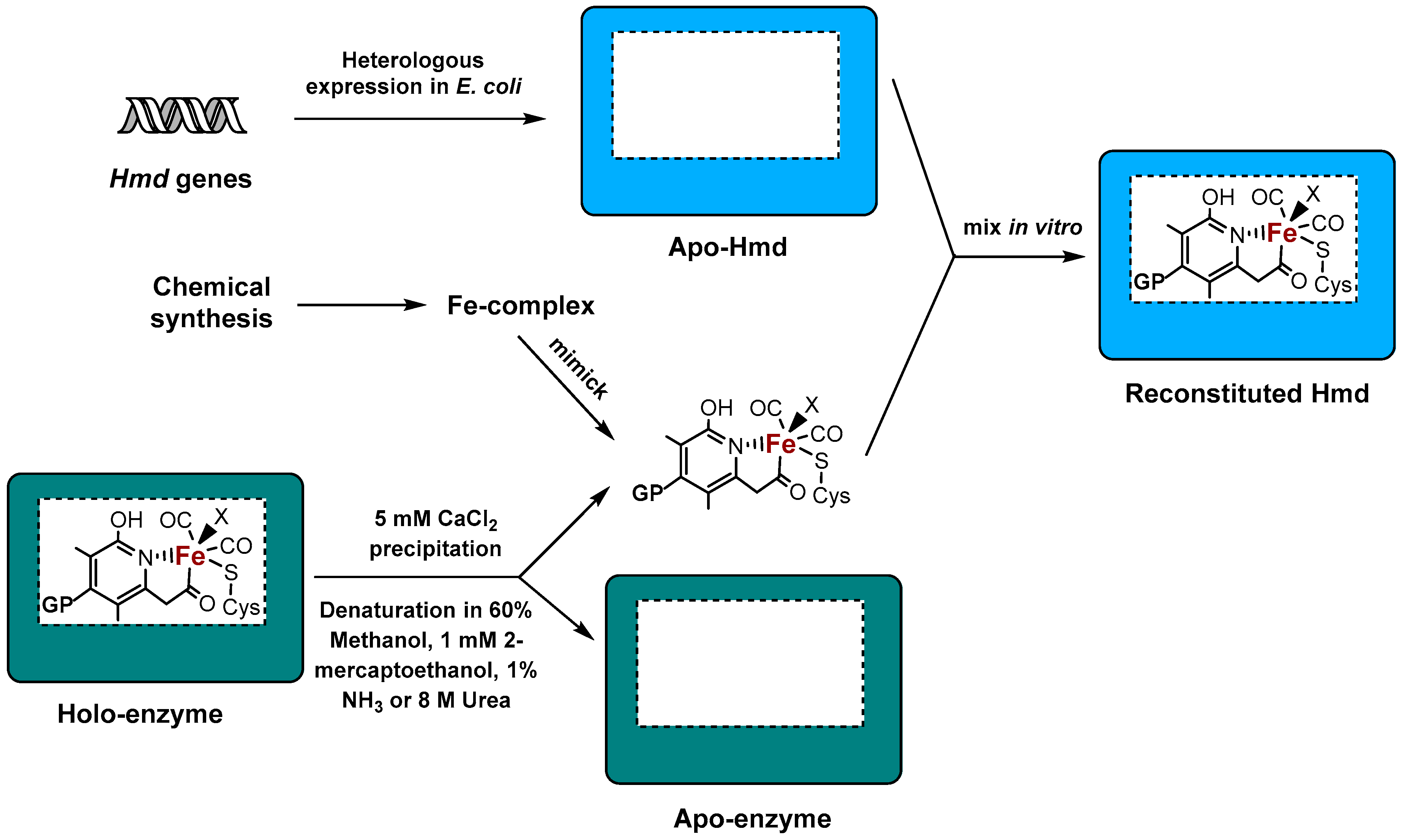
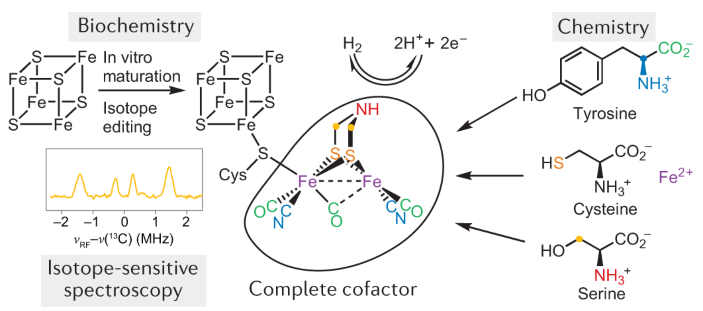
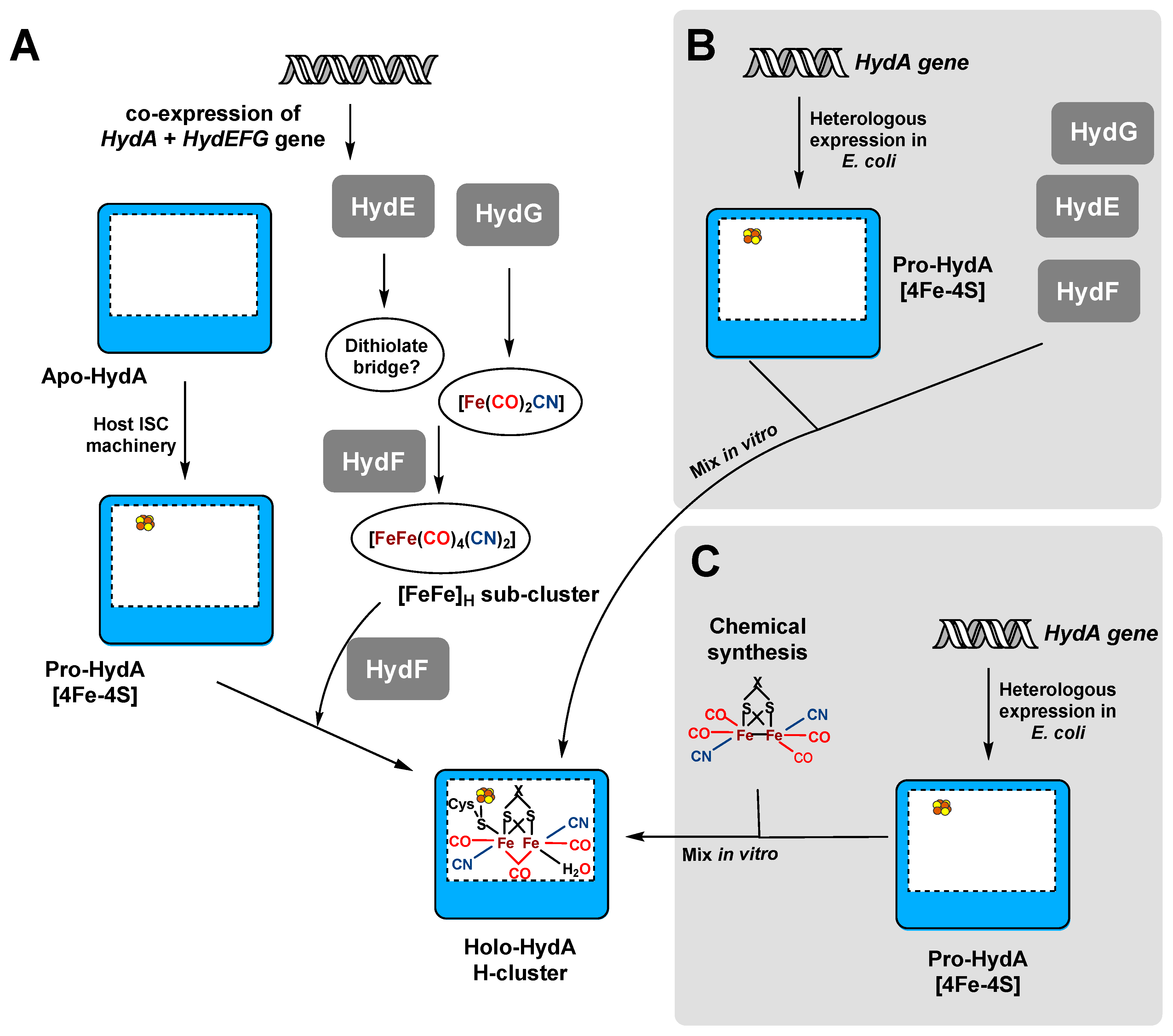
Hydrogenases are metalloenzymes that catalyze the reversible reduction of protons at unusual metal centers This Current Topic discusses recent advances in elucidating the steps involved in the biosynthesis of the complex metal cluster at the FeFehydrogenase (HydA) active site, known as the HclusterThe hydrogenases containing a diiron center with a bridging dithiolate cofactor are called FeFe hydrogenases Three families of FeFe hydrogenases are recognized cytoplasmic, soluble, monomeric hydrogenases, found in strict anaerobes such as Clostridium pasteurianum and Megasphaera elsdenii They catalyse both H 2 evolution and uptake




The Crystal Structure Of Fe Hydrogenase Reveals The Geometry Of The Active Site Science




Hydrogenase Wikipedia




2 Molecular Structure Of Fefe Hydrogenase Cofactor Metal Ions Are Download Scientific Diagram



Ijms Free Full Text Heterologous Hydrogenase Overproduction Systems For Biotechnology An Overview Html




Scielo Brasil Electrocatalysis By Hydrogenases Lessons For Building Bio Inspired Device Electrocatalysis By Hydrogenases Lessons For Building Bio Inspired Device




Insights Into Fefe Hydrogenase Structure Mechanism And Maturation Sciencedirect




Structure Of The H Cluster In Fefe Hydrogenase From Chlamydomonas Download Scientific Diagram



Evolution In The Understanding Of Fe Hydrogenase Chemical Society Reviews Rsc Publishing




A Model Of The Fefe Hydrogenase Active Site With A Biologically Relevant Azadithiolate Bridge A Spectroscopic And Theoretical Investigation Erdem 11 Angewandte Chemie International Edition Wiley Online Library
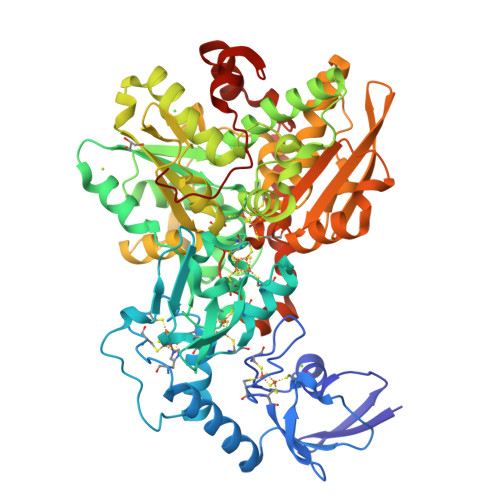



Rcsb Pdb 4xdd Apo Fefe Hydrogenase Cpi



Nasa Astrobiology Institute



H Cluster Assembly During Maturation Of The Fefe Hydrogenase Springerlink




Ultrafast Electron Transfer From Cdse Quantum Dots To A Fefe Hydrogenase Mimic Physical Chemistry Chemrxiv Cambridge Open Engage



1




Fefe Hydrogenase Maturation H Cluster Assembly Intermediates Tracked By Electron Paramagnetic Resonance Infrared And X Ray Absorption Spectroscopy Springerlink




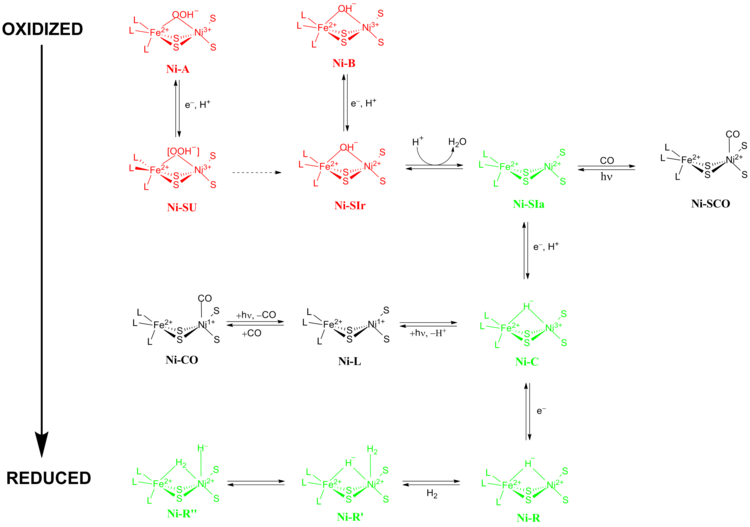
Electrocatalytic Mechanism Of Reversible Hydrogen Cycling By Enzymes And Distinctions Between The Major Classes Of Hydrogenases Pnas
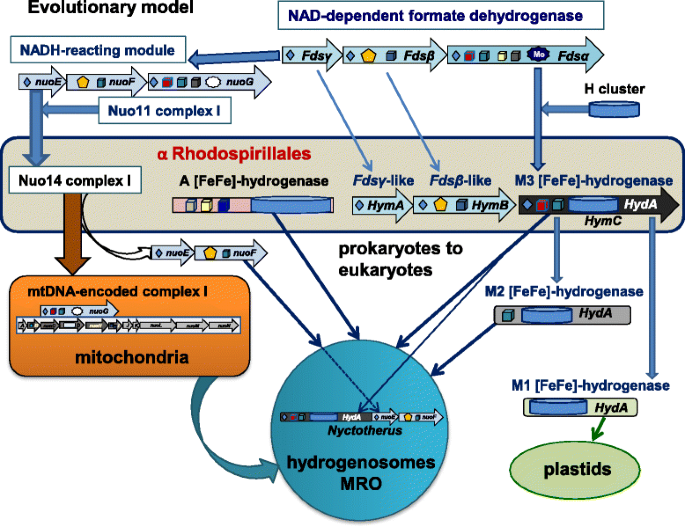



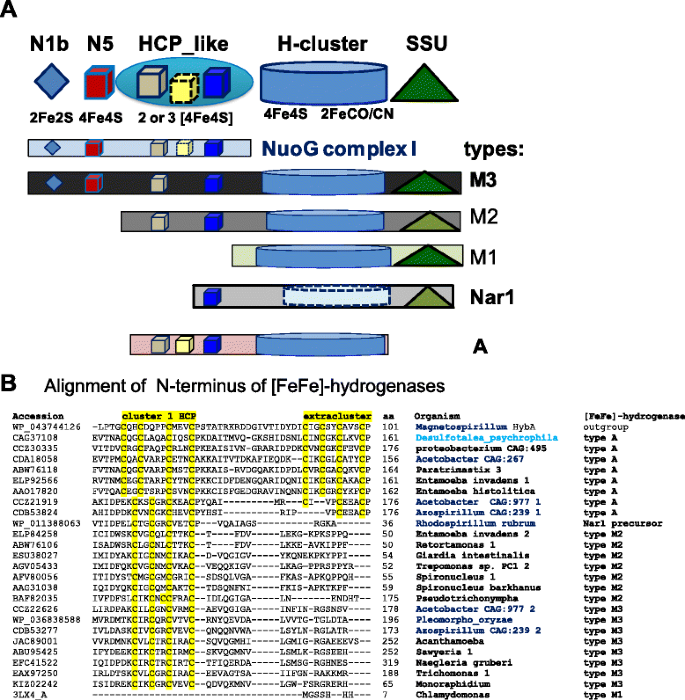
Alpha Proteobacterial Ancestry Of The Fe Fe Hydrogenases In Anaerobic Eukaryotes Biology Direct Full Text




The Crystal Structure Of Fe Hydrogenase Reveals The Geometry Of The Active Site Science



Mechanism Of The Biosynthesis Of Carbon Monoxide Necessary For The Expression Of The Metalloenzyme Activity Press Release Spring 8 Web Site



Ethz Ch Content Dam Ethz Special Interest Chab Physical Chemistry Reiher Dam Images Posters Grc Finkelmann12 Pdf




A Radical Intermediate In Tyrosine Scission To The Co And Cn Ligands Of Fefe Hydrogenase Science



Delocalized Molecular Orbitals Of The 6fe6s Cluster Of The Fefe Hydrogenase Stanford Synchrotron Radiation Lightsource



A Safety Cap Protects Hydrogenase From Oxygen Attack Nature Communications



Www Mdpi Com 73 4344 11 2 238 Pdf



Alpha Proteobacterial Ancestry Of The Fe Fe Hydrogenases In Anaerobic Eukaryotes Biology Direct Full Text




Hydrogenase Wikiwand




15 A Schematic Representation Of The Crystal Structures Download Scientific Diagram




Pdf Fefe Hydrogenases Recent Developments And Future Perspectives Semantic Scholar




Ppt Ni Fe Hydrogenase Powerpoint Presentation Free Download Id




Proposed Mechanism For Reversible Proton Reduction Catalyzed By The Download Scientific Diagram



Fefe Hydrogenases Recent Developments And Future Perspectives Chemical Communications Rsc Publishing




Characterization Of Fefe Hydrogenase O2 Sensitivity Using A New Physiological Approach Journal Of Biological Chemistry
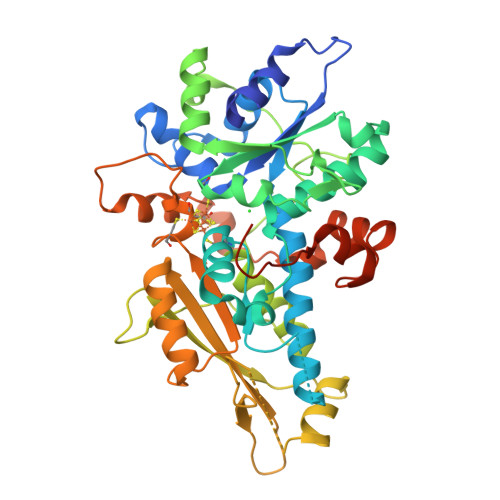



Rcsb Pdb 6gm7 Fefe Hydrogenase Hyda1 From Chlamydomonas Reinhardtii Variant E144a




Proposed Mechanism For Reversible Proton Reduction Catalyzed By The Download Scientific Diagram



Fefe Hydrogenases Maturation And Reactivity Of Enzymatic Systems And Overview Of Biomimetic Models Chemical Society Reviews Rsc Publishing
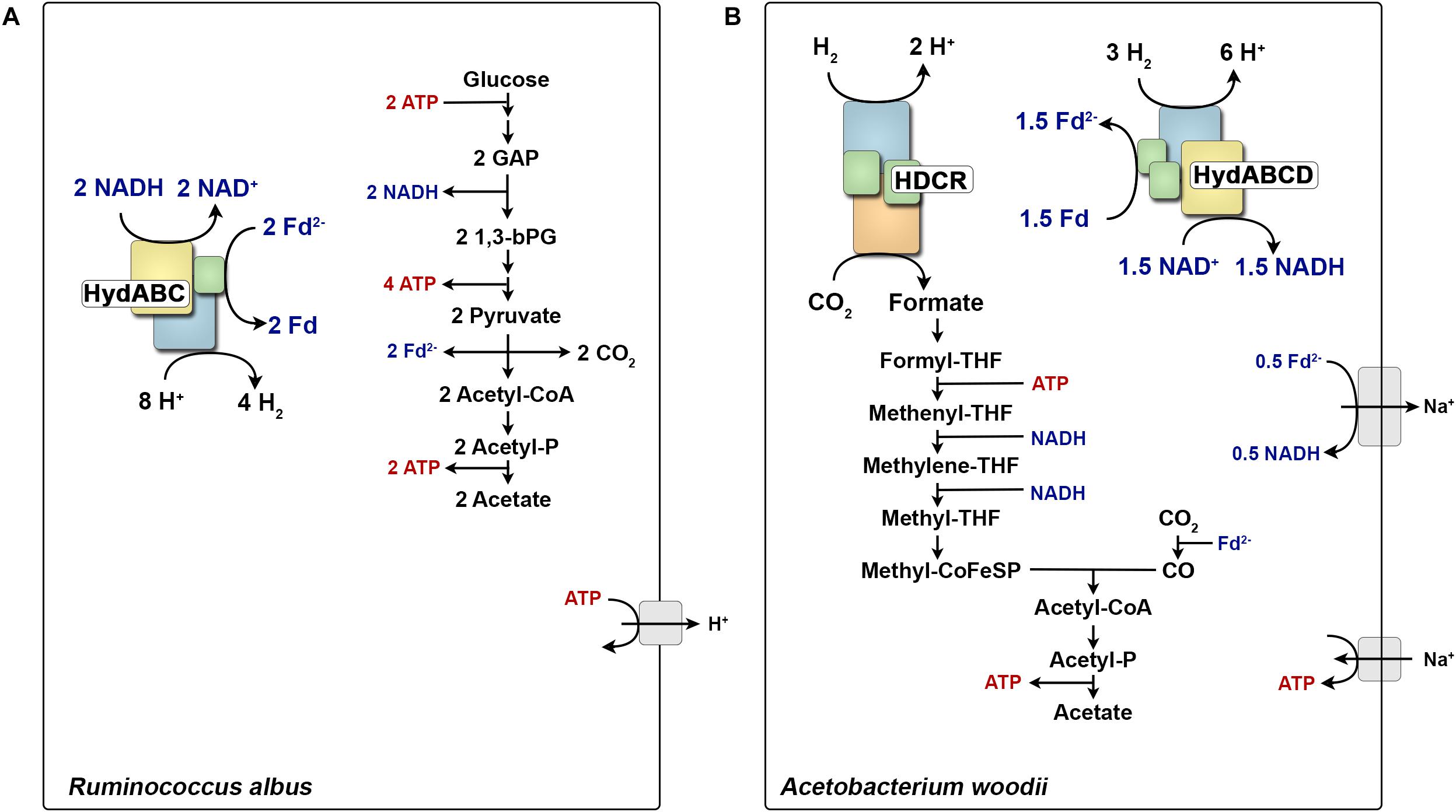



Frontiers Complex Multimeric Fefe Hydrogenases Biochemistry Physiology And New Opportunities For The Hydrogen Economy Microbiology
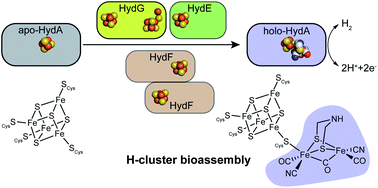



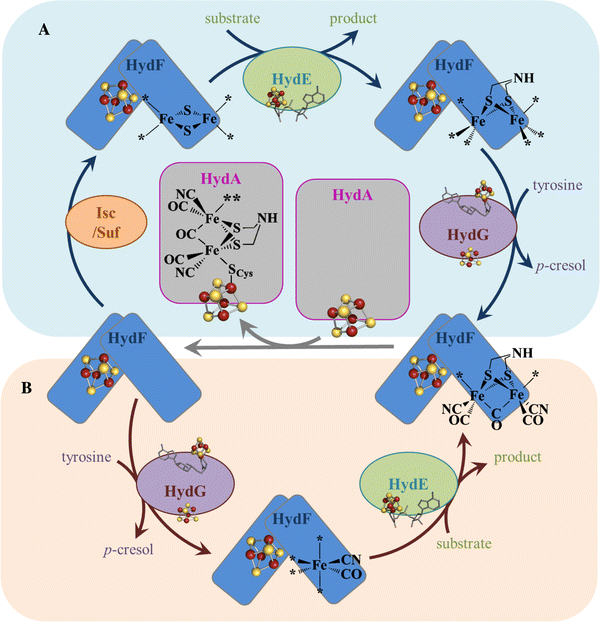
Biosynthesis Of The Catalytic H Cluster Of Fefe Hydrogenase The Roles Of The Fe S Maturase Proteins Hyde Hydf And Hydg Chemical Science Rsc Publishing




Fig 2 The Molecular Structure Of The Fefe Hydrogenase Active Site The H Cluster The Molecular Structure Of The Fefe Hydrogenase Active Site The Ppt Download




Stepwise Isotope Editing Of Fefe Hydrogenases Exposes Cofactor Dynamics Pnas




The Beta Subunit Of Non Bifurcating Nadh Dependent Fefe Hydrogenases Differs From Those Of Multimeric Electron Bifurcating Fefe Hydrogenases Abstract Europe Pmc




Co And Cn Syntheses By Fefe Hydrogenase Maturase Hydg Are Catalytically Differentiated Events Pnas




Fefe And Nife Hydrogenase Diversity Mechanism And Maturation Sciencedirect




6 A Crystal Structure Of The Fefe Hydrogenase From Desulfovibrio Download Scientific Diagram



Bioassembly Of Complex Iron Sulfur Enzymes Hydrogenases And Nitrogenases Nature Reviews Chemistry



Hydrogenases




Skeletal Representation Of The Catalytic Cycle Of The Fefe Download Scientific Diagram




Active Site Of Fe Fe Hydrogenase Enzyme X Nh Or Ch 2 Download Scientific Diagram




18 Active Site Biochemistry Of Fefe Hydrogenase Enzyme Justice Download Scientific Diagram



2



Hydrogenases




Atomic Resolution Modeling Of The Ferredoxin Fefe Hydrogenase Complex From Chlamydomonas Reinhardtii Biophysical Journal



Ijms Free Full Text Heterologous Hydrogenase Overproduction Systems For Biotechnology An Overview Html



Cell Free H Cluster Synthesis And Fefe Hydrogenase Activation All Five Co And Cn Ligands Derive From Tyrosine




Oxygen Stable State Of Fefe Hydrogenase Studied Chemviews Magazine Chemistryviews




Molecular Basis Of Fefe Hydrogenase Function An Insight Into The Complex Interplay Between Protein And Catalytic Cofactor Sciencedirect
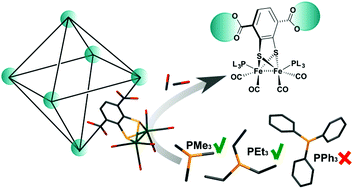



Fefe Hydrogenase Active Site Model Chemistry In A Uio 66 Metal Organic Framework Chemical Communications Rsc Publishing



Fe Only Hydrogenase Chemistry Libretexts




Spectroscopic Investigations Under In Vivo Conditions Reveal The Complex Metal Hydride Chemistry Of Fefe Hydrogenase Biological And Medicinal Chemistry Chemrxiv Cambridge Open Engage



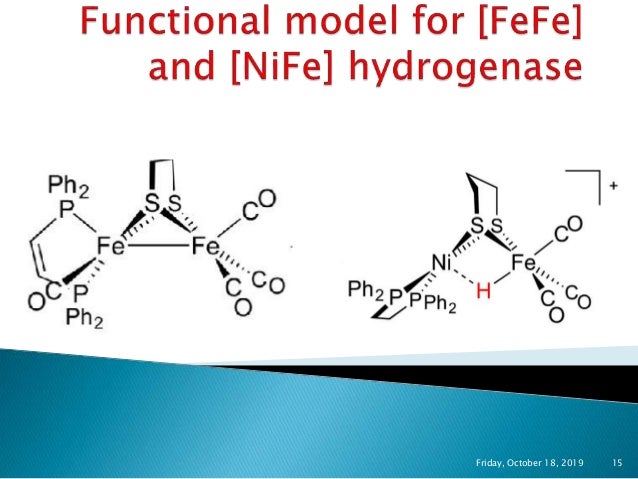
Nife Hydrogenase Wikipedia



Hydrogenase



Hydrogenase Inspired Catalysts Lichtenberger Research Group



Hydrogenases
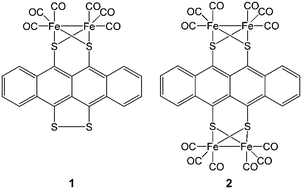



Electrocatalytic Hydrogen Production Using Fefe Hydrogenase Mimics Based On Tetracene Derivatives New Journal Of Chemistry Rsc Publishing
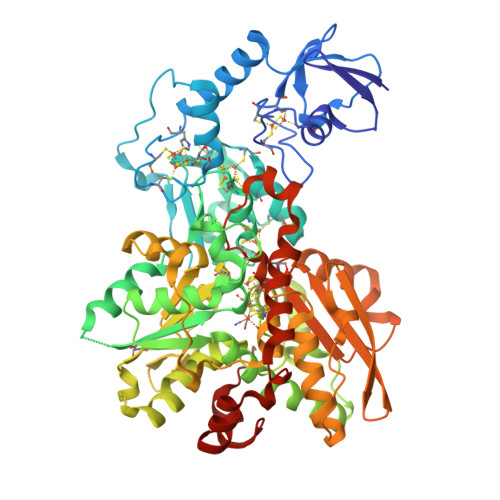



Rcsb Pdb 6n59 1 0 Angstrom Crystal Structure Of Fefe Hydrogenase
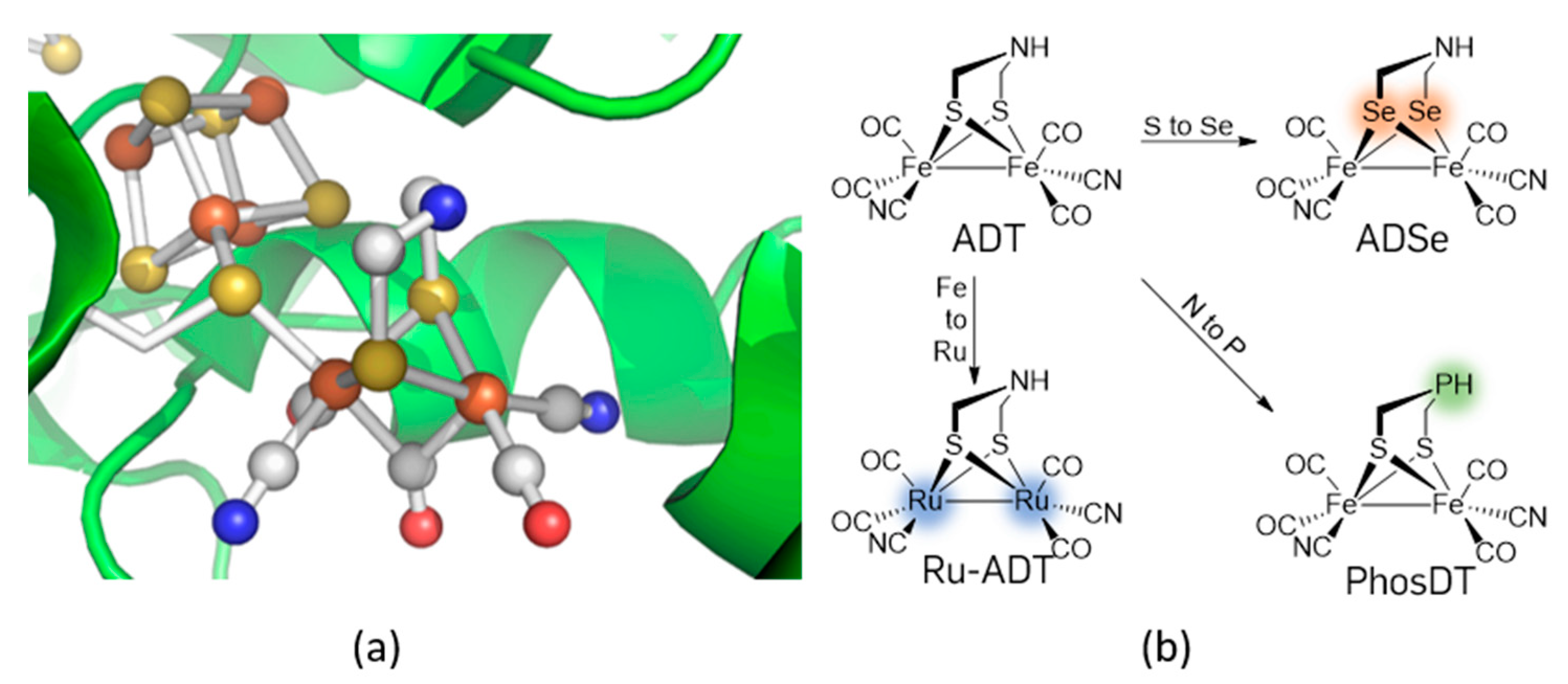



Catalysts Free Full Text New Phosphorous Based Fefe Hydrogenase Models
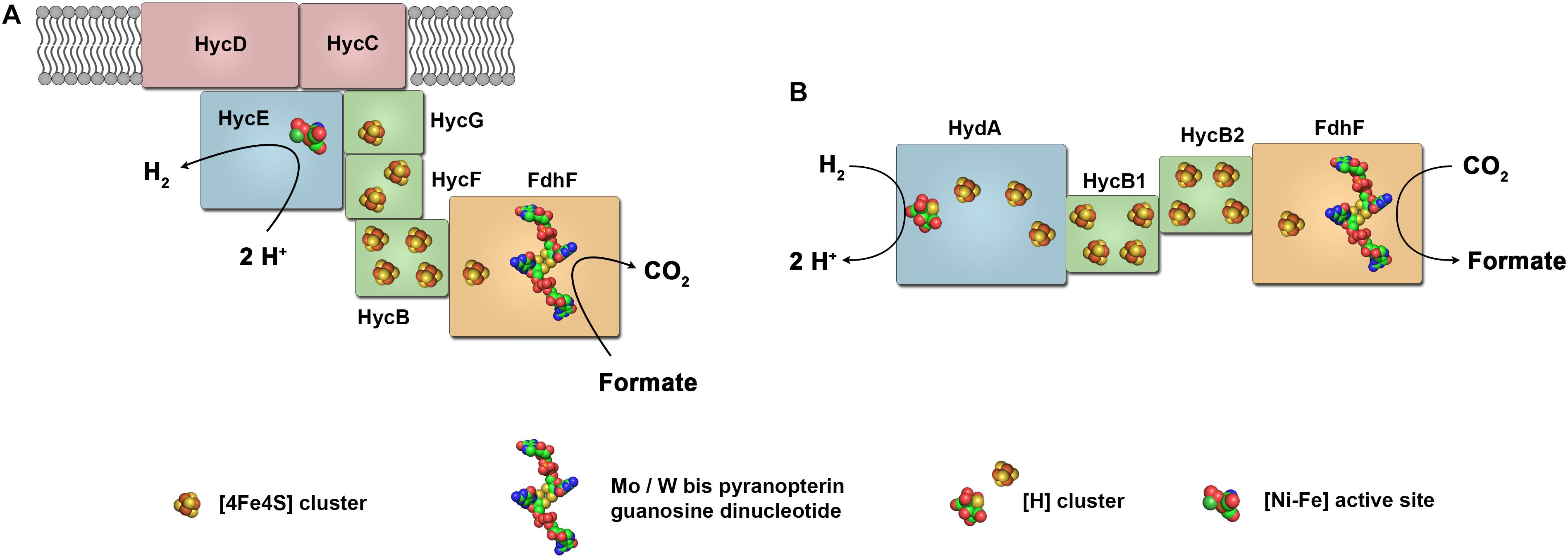



Frontiers Complex Multimeric Fefe Hydrogenases Biochemistry Physiology And New Opportunities For The Hydrogen Economy Microbiology



1



1
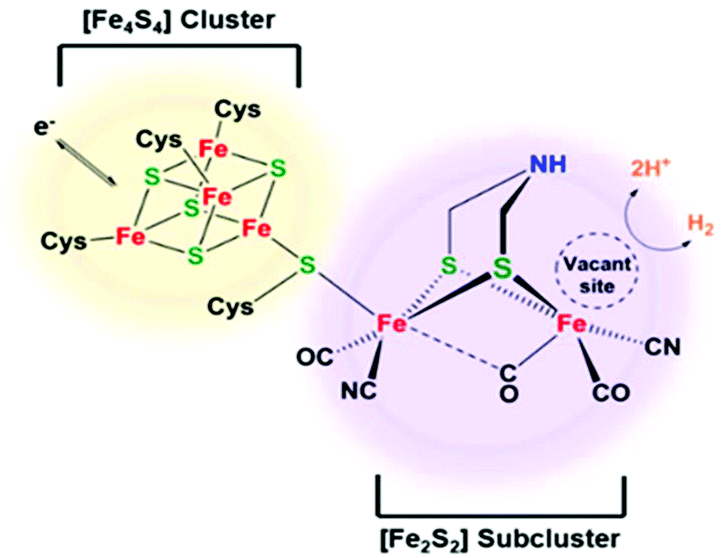



Fefe Hydrogenase H Cluster Mimics Mediated By Naphthalene Monoimide Derivatives Of Peri Substituted Dichalcogenides Dalton Transactions Rsc Publishing
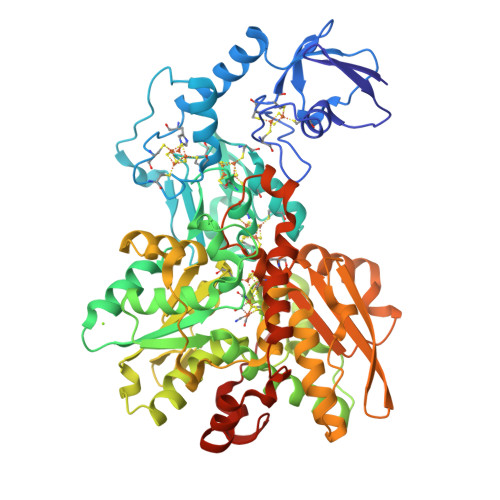



Rcsb Pdb 5la3 Fefe Hydrogenase Cpi From Clostridium Pasteurianum Variant E279a




The Active Site Structure Of Fefe Hydrogenase Download Scientific Diagram




Fefe And Nife Hydrogenase Diversity Mechanism And Maturation Sciencedirect




Nife Fefe And Fe Hydrogenase Models From Isomers Science Advances




Research Projects Department Of Chemistry Angstrom Uppsala University Sweden




Figure 4 From Sulfide Protects Fefe Hydrogenases From O2 Semantic Scholar
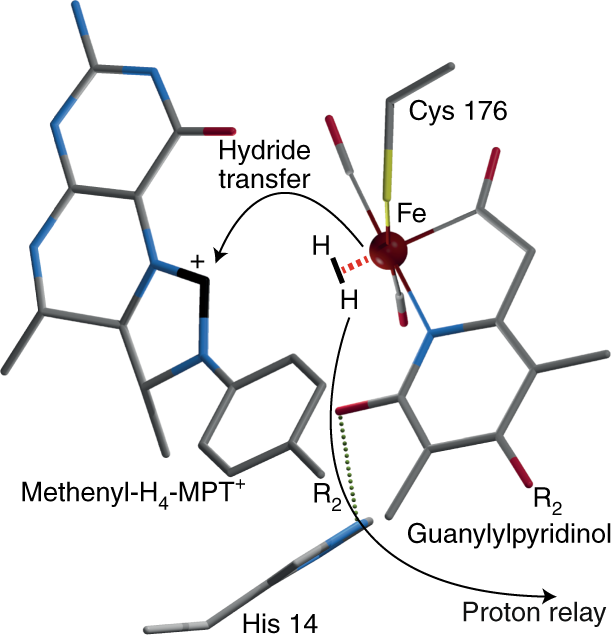



How Fe Hydrogenase Metabolizes Dihydrogen Nature Catalysis



0 件のコメント:
コメントを投稿